Introduction
In pharmaceutical manufacturing, visual inspection is a critical quality control measure that has traditionally relied on human inspectors. However, human visual inspection comes with inherent limitations. Inspectors face fatigue during extended inspection sessions, which can lead to inconsistent results, and studies show significant variability between different inspectors examining the same products. Human visual detection also has probabilistic limitations, particularly for small, subtle, or complex defects, with most defect types never reaching 100% detection probability regardless of inspector experience.
These challenges are magnified when dealing with difficult-to-inspect products like molded glass vials, lyophilized cakes, or translucent containers. Unsupervised machine learning (ML) offers a fundamentally different approach to traditional visual inspection because it learns from normal samples rather than focusing on defects. At the 2025 PDA conference, Boon Logic presented this methodology and how it addresses the inherent limitations of human inspection.
How Human Visual Perception Works
To develop effective automated inspection systems, we must first understand how humans detect anomalies. Human visual perception operates on three primary axes: size, contrast, and novelty.
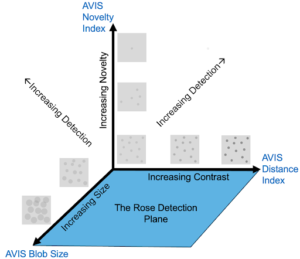
Size
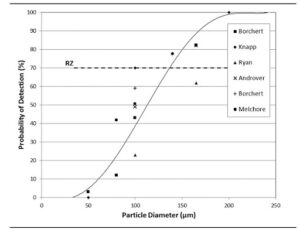
As particle size increases, the probability of detection improves. According to established research, a 70% detection rate typically requires particles of approximately 130–140 microns in diameter. This relationship is represented as an S-curve, where detection probability increases with particle size.
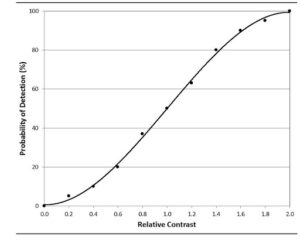
Contrast
While size receives significant attention in pharmaceutical inspection standards, contrast is equally important. A 500-micron particle with no contrast against its background will remain invisible to inspectors. Testing reveals that humans typically require a contrast level greater than 8 grayscale values (on a 0–255 scale) to reliably detect anomalies.
Novelty
The third and often overlooked dimension of perception is novelty. According to predictive coding theory, our brains continuously build unconscious prediction models of what we expect to see. When visual input deviates significantly from this expectation, the novelty draws our attention. This explains why unusual features in otherwise familiar objects stand out, even when their size or contrast might not be exceptional.
The Rose Detection Model
Albert Rose, a physicist who worked for RCA in the mid-20th century, developed what’s now known as the Rose Detection Model. Though originally focused on television technology, his research has profound implications for pharmaceutical inspection. Rose determined that humans can detect small objects against backgrounds when the object’s contrast is at least five times the background noise standard deviation. This principle in visual perception with television cameras and screens also applies to automated inspection systems that aim to match or exceed human performance.
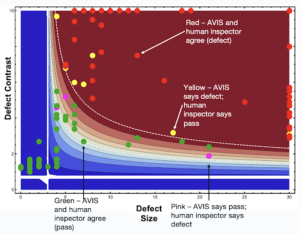
Unsupervised Machine Learning in Visual Inspection
Unsupervised ML offers a fundamentally different approach to automated visual inspection (AVI) compared to traditional rule-based systems or supervised ML approaches.
Training from Compliant Units
Rather than requiring extensive libraries of labeled defect images, unsupervised ML models learn from images of compliant vials, which are abundantly available in Good Manufacturing Practice (GMP) environments.
No Image Labeling Required
The unsupervised approach removes one of the most significant barriers to implementing AI-based inspection: the need for image labeling. Large pharmaceutical companies have built entire platforms just to manage their image labeling processes, making this approach cost-prohibitive for most companies
Straightforward Model Maintenance
When suppliers or components change, supervised ML models may require complete retraining with new defect libraries. With unsupervised approaches, adaptation requires only a new set of compliant units, making model maintenance significantly more manageable.
Superior Detection of Unknown Defects
In addition, unsupervised ML excels at detecting new or unknown defects. While supervised approaches can only detect defects represented in their training sets, unsupervised models flag anything that deviates significantly from normal patterns, regardless of whether that specific defect type was anticipated.
AI-Based Inspection in High Dimensions
A typical AI-based visual inspection model operates in much higher dimensions than humans can visualize. While we can conceptualize three-dimensional models, AI-based inspection recipes might operate in a 64-dimensional space containing thousands of clusters describing the normal variation of compliant vials.
Case Study: Translucent Plastic Syringes
AVIS, Boon Logic’s AI-based visual inspection platform that uses unsupervised machine learning, recently completed a feasibility study for translucent plastic syringes. In the case study, the client was faced with the following inspection challenges:
- Particles often cling to inner sidewalls or grommet faces
- Bubbles form on sidewalls and grommet faces
- Moisture between grommet ribs has low contrast
- Packing materials sometimes adhere to exterior surfaces
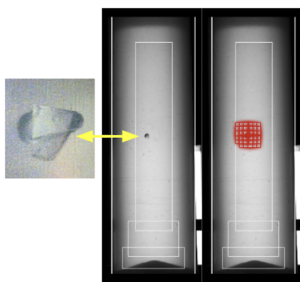
In the case study, AVIS successfully distinguished between normal variations—such as common bubbles, which have a low novelty index—and true defects. In one notable instance, a human inspector misidentified a 2,000-micron plastic fragment as a bubble, but it was later correctly detected by AVIS. This example highlights how machine learning can augment human capabilities rather than merely replicating them.
Conclusion
Understanding how humans detect visual anomalies provides the foundation for developing more effective automated inspection systems. By incorporating the three dimensions of human visual perception—size, contrast, and novelty—unsupervised ML approaches can not only match human capabilities but exceed them in specific areas.
This approach is particularly valuable for difficult-to-inspect products, where traditional AVI systems struggle with high false eject rates. By focusing on learning normal variation rather than cataloging defects, AVIS can adapt more quickly to new products, is more easily maintained after manufacturing changes, and can detect novel defects that might otherwise escape notice.
As pharmaceutical manufacturing continues to evolve toward increasing complexity and higher quality standards, understanding these principles of visual perception and applying them through advanced machine learning will be essential for maintaining product safety while improving manufacturing efficiency.